This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
Acute lymphoblastic leukemia

doktorandka Katka Bérešová
Acute lymphoblastic leukemia is one of the most common among childhood malignancies. Despite the severity of the disease, treatment of childhood leukemia is one of the major medical success stories of modern medicine. This once uniformly fatal disease is currently treatable in up to 90 % of cases. However, treatment is difficult for pediatric patients not only physically but also mentally. It is accompanied by a number of lifelong health complications while it cannot always prevent relapse, the return of the disease. In many cases, this is already too much of a burden on the child’s body.
Development of acute lymphoblastic leukemia
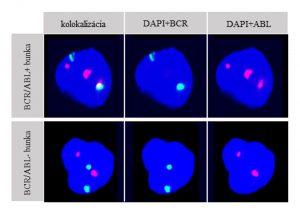
Pict. 1 Characteristic image of a nucleus negative for BCR/ABL PFG and a nucleus positive for BCR/ABL PFG: 3 spectrums (spectrum blue (DAPI), spectrum red (ABL), spectrum green (BCR)) are shown. A nucleus positive for reciprocal BCR/ABL translocation is characterised with 3 red fluorescent signals, one of which fuses with one green signal and together they produce a yellow fluorescent signal.The negative nucleus contains 2 green and 2 red non-colocazing signals.
The onset of childhood acute leukemia has not been fully elucidated. The generally accepted theory is based on a model of two hits. The first hit occurs as early as in the prenatal stage of the childs development, most often it is represented by chromosomal translocation, i.e. the exchange of a section of DNA between two non-homologous chromosomes. The result is a formation of preleukemic fusion genes with new or altered function. These include TEL/AML1, BCR/ABL or MLL/AF9, and these rearrangements are often present in the patient’s leukemic blast cells at the time of diagnosis. However, the fusion gene alone is not sufficient for the development of leukemia, further hits in the postnatal period are necessary. These are often point mutations, small changes near the fusion gene that cause its expression, oncoprotein formation and leukemic blast formation.

PhD. student Katka Bérešová during awarding the JCI-Slovakia Award for the greatest potential contribution to society at the academy year 2018/2019
What does the PhD. student Katka Bérešová do, who was awarded the JCI-Slovakia Award for the greatest potential contribution to society at the academy year 2018/2019
In our laboratory at the Department of Radiobiology, CRI BMC SAS under the leadership of doc. Igor Beliaev, we examine peripheral blood, bone marrow, as well as rare samples of frozen umbilical cord blood of leukemic patients. We are interested in the question of whether a specific subpopulation of hematopoietic stem and progenitor cells is responsible for the onset and return of leukemia. First, mononuclear blood cells are divided into different subpopulations of hematopoetic stem cells by FACS according to a combination of different surface CD markers. Individual subpopulations are then analyzed for the presence of preleukemic fusion genes by Fluorescence in situ hybridization using special dual-fusion and break-apart DNA fluorescence probes.
Identification of such a subpopulation would not only allow to assess the risk of leukemia onset in neonates, but also allow the design of a more specific immunotherapy treatments. Moreover, screening of umbilical cord blood used in hematopoetic stem cell transplantaion for preleukemic stem cells and its degradation would prevent the formation of rare but fatal form of donor-cell leukemia.








