This website uses cookies so that we can provide you with the best user experience possible. Cookie information is stored in your browser and performs functions such as recognising you when you return to our website and helping our team to understand which sections of the website you find most interesting and useful.
The results of a seroepidemiological study of SAS employees

These days, the Biomedical Research Center SAS has published findings from a cross-sectional seroprevalence study focused on the presence of specific IgG antibodies against the SARS-Co-2 virus produced as a result of overcoming COVID-19 diseases and/or vaccination. The study was carried out among the employees of the Slovak Academy of Sciences during the months of June to August 2021, and its aim was to determine the occurrence and relative level of the SARS-CoV-2 virus in the SAS environment, to inform research institutions about the situation in their workplaces and to assess the risk of the effects of possible SARS-CoV-2 infection with a perspective to guiding decisions on institutional measures to maintain research in connection with the development of the epidemic situation.
Study participants provided informed consent, anamnestic information and dry blood drop samples, which were analysed by ELISA method. The relative levels of specific antibodies found in 1928 individuals showed a seroprevalence of 84.13% and led to the following main conclusions:
- mRNA-based vaccines elicit a better antibody response compared to adenovirus-based vaccines
- the levels of antibodies produced reflect the severity of the symptoms of COVID-19
- vaccination after overcoming COVID-19 significantly increases the level of IgG antibodies, especially in the asymptomatic and mild course of the disease
- antibody levels decrease with increasing time from vaccination or overcoming COVID-19
In seroprevalence, differences were observed not only between groups of employees from individual departments of sciences of SAS but also from individual organisations. Workplaces with relatively high seropositivity and participation rates have the potential to provide a safer work environment compared to workplaces where seroprevalence has been low or unknown due to low participation.
The study’s findings may have an impact on management decisions during the further development of the pandemic, bearing in mind that immunity weakens over time and that the more contagious Delta variant of SARS-CoV-2 is currently spreading. However, the results of the study offer only a partial view of adaptive immunity to natural SARS-CoV-2 infection and/or vaccination. T cell-mediated immunity is also known to play an important role in antiviral defence, and even in individuals with low antibody levels, effector and memory T cells capable of effective defence response to COVID-19 may develop after vaccination or infection.
The full article entitled “Seroprevalence of SARS-CoV-2 IgG antibodies in the staff of the Slovak Academy of Sciences in response to COVID-19 and/or vaccination: situation in August 2021“ is available HERE.
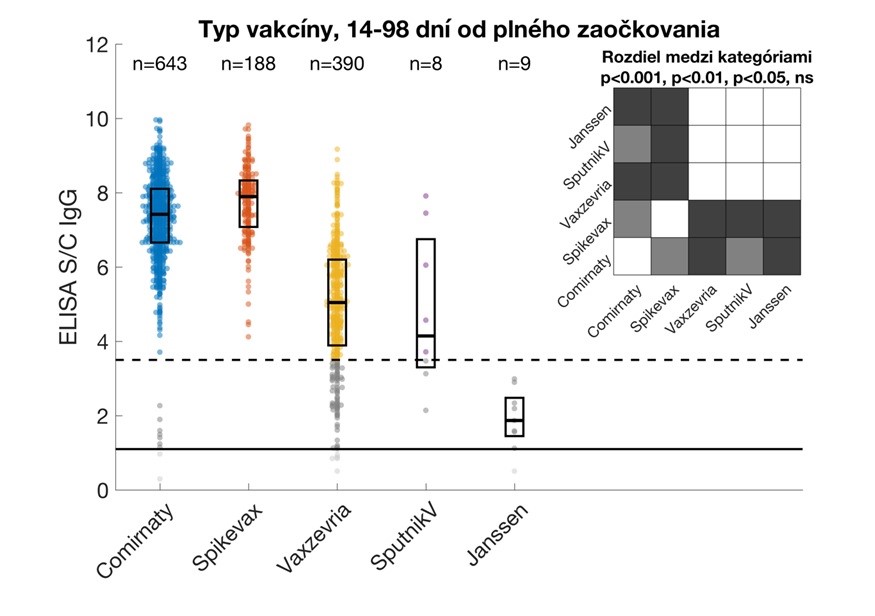
Relative IgG levels against the S1 subunit of the SARS-CoV-2 protein in study participants vaccinated with the given vaccines. Data are from participants who provided samples 14 – 98 days from the date of their full vaccination. Values are expressed as a signal to calibrator (S/C) ratio, with a positivity limit of 1.1 (solid horizontal line) and a high positivity limit of 3.5 (dashed horizontal line). The median and interquartile ranges are shown. The upper right corner shows statistically significant differences between vaccine types. Comirnaty (Pfizer / BioNTech), Spikevax (Modern), Vaxzevria (Astra Zeneca/Oxford University).
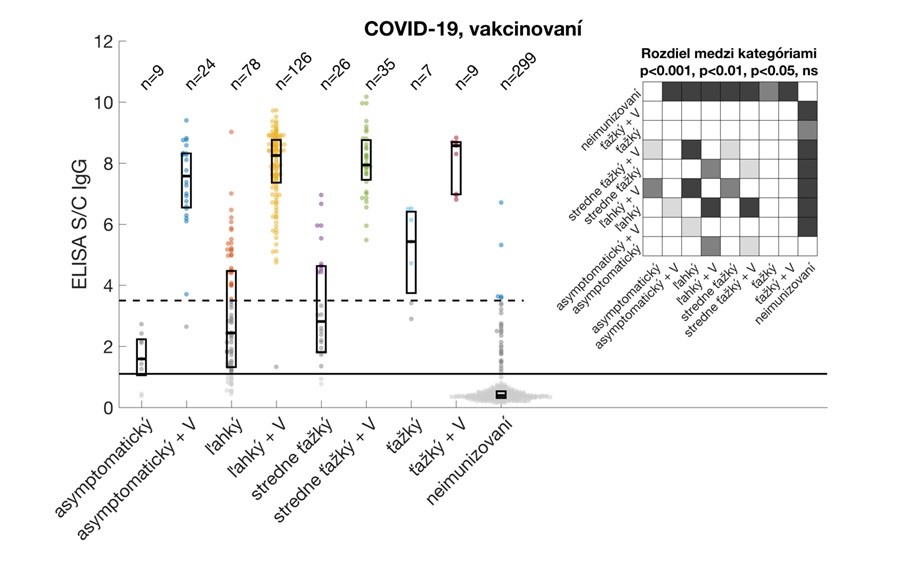
Relative antibody levels in study participants after overcoming COVID-19 and/or vaccination. Participants were stratified by vaccine dose (regardless of vaccine type) without or with prior COVID-19. Data are expressed as a signal to calibrator (S/C) ratio, with a positivity limit 1.1 (solid horizontal line) and a high positivity limit of 3.5 (dashed horizontal line). The median and interquartile ranges are shown. The upper right corner shows statistically significant differences between the individual groups analysed.
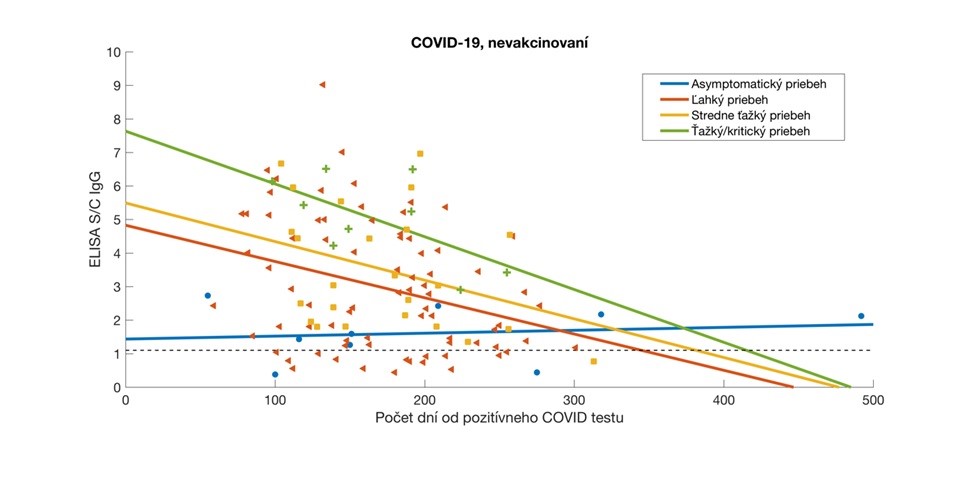
Relative antibody levels in participants who overcame COVID-19 were analysed relative to the time elapsed since their positive RT-PCR or Ag test. Participant data were stratified according to the severity of COVID-19 symptoms and the corresponding regression lines are colour-coded. Antibody values are expressed as a signal to calibrator (S/C) ratio, with a positivity limit of 1.1 (dashed horizontal line).
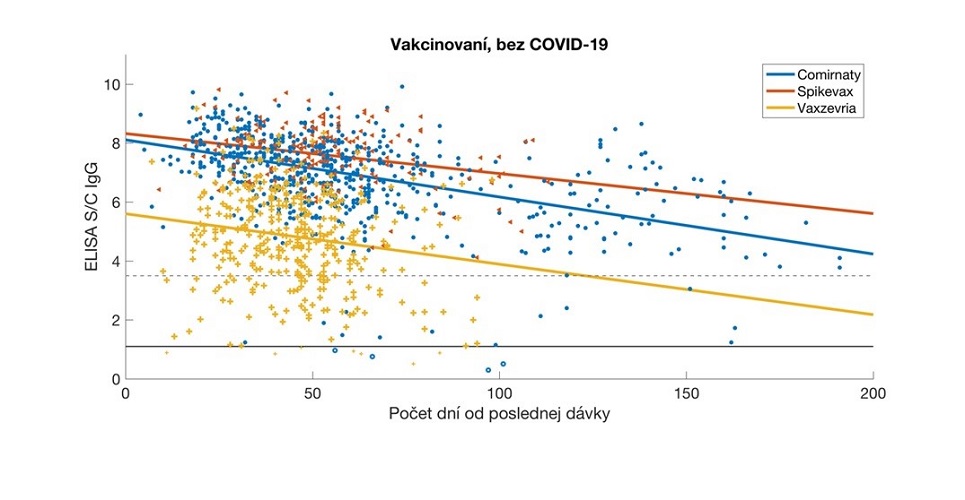
Relative antibody levels in serum samples from vaccinated participants, analysed in relation to the time elapsed since the date of their full vaccination. The data were stratified by vaccine type and the corresponding regression lines are colour-coded. Antibody values are expressed as a signal to calibrator (S/C) ratio, with a positivity limit of 1.1 (solid horizontal line) and a high positivity limit of 3.5 (dashed horizontal line). Comirnaty (Pfizer / BioNTech), Spikevax (Modern), Vaxzevria (Astra Zeneca/Oxford University).
Text and photo in text: BMC SAS
Translated by: SAS
Introductory photo: Martin Bystriansky, SAV








